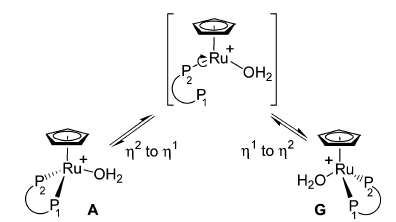
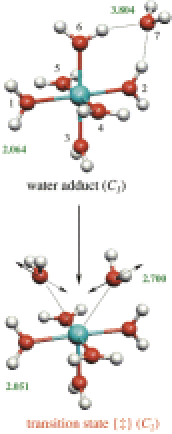
-
Calculated volume and energy profiles for water exchange on t2g6 rhodium(III) and iridium(III) hexaaquaions : conclusive evidence for an Ia mechanism
D. De Vito, J. Weber and A.E. Merbach
Inorganic Chemistry, 43 (3) (2004), p858-864


DOI:10.1021/ic035096n | unige:3325 | Abstract | Article HTML | Article PDF
An Ia mechanism was assigned for water exchange on the hexaaquaions Rh(OH2)63+ and Ir(OH2)63+ on the basis of negative ΔV‡ experimental values (−4.2 and −5.7 cm3 mol-1, respectively). The use of ΔV‡ as a mechanistic criterion was open to debate primarily because ΔV‡ could be affected by extension or compression of the nonparticipating ligand bond lengths on going to the transition state of an exchange process. In this paper, volume and energy profiles for two distinct water exchange mechanisms (D and Ia) have been computed using quantum chemical calculations which include hydration effects. The activation energy for Ir(OH2)63+ is 32.2 kJ mol-1 in favor of the Ia mechanism (127.9 kJ mol-1), as opposed to a D pathway; the value for the Ia mechanism being close to ΔH‡ and ΔG‡ experimental values (130.5 kJ mol-1 and 129.9 kJ mol-1 at 298 K, respectively). Volumes of activation, computed using Connolly surfaces and for the Ia pathway (ΔV‡calc = −3.9 and −3.5 cm3 mol-1, respectively, for Rh3+ and Ir3+), are in agreement with the experimental values. Further, it is demonstrated for both mechanisms that the contribution to the volume of activation due to the changes in bond lengths between Ir(III) and the spectator water molecules is negligible: −1.8 for the D, and −0.9 cm3 mol-1 for Ia mechanism. This finding clarifies the debate about the interpretation of ΔV‡ and unequivocally confirms the occurrence of an Ia mechanism with retention of configuration and a small a character for both Rh(III) and Ir(III) hexaaquaions.